Using air viscosity (μ) of 185 × 10- 5 N s/m 2 at 25 °C, an entrance velocity ( v0) of 5.3 cm/s, a PP web with a thickness of 0.3 mm and basis weight of 30 gsm, a pressure drop of the air across the web of 2.8 mmH 2 O, and a PP having a polymer density of 0.91 g/cm 3, Eq. 2.2 can be used to calculate the porosity of the web as 89%.
- The kinematic viscosity is the dynamic viscosity divided by the density of the fluid. In cgs units the unit is called the centistoke. In cgs units the unit is called the centistoke. The viscosity of water decreases smoothly from the freezing point while the density remains essentially constant except for the small maximum in density near 4°C.
- The value of the dynamic viscosity coefficient is found to be a constant with pressure but the value depends on the temperature of the gas. Sutherland provides an equation for the dependence on temperature T: mu = mu0. ((T / T0)^1.5). ((T0 + 198.72) / (T + 198.72)).
We all have a feel for viscosity. More viscous a fluid moredifficult for it to flow. Oils flow at a slower rate than water.We understand viscosity as a property that tends to retard fluidmotion.But we do have a more rigorous definition of viscosity,which can be developed from the thought experiment describedbefore. It was seen that when a shear force is applied to thetop plate the fluid undergoes a continuous deformation( What is a Fluid? Fig.1.4). As a result the block of fluidabcd deforms to ab'c'd after a time t. Letthe speed of the top plate be U. It is an important property offluids that the layer of fluid adjacent to a solid surface moveswith the same velocity as the solid surface. This is called the'No Slip' condition. Accordingly fluid layer closer to the topplate moves with a speed U while that closer to the lower plate isat rest. Thus the velocity of the fluid varies continuously fromzero on the lower plate to U at the upper plate. In other words avelocity gradient develops in the fluid. In the simple case of theflow between parallel plates this is a linear profile. Thevelocity gradient is given by
where h is the distance between the two plates. In a small instant of time we find that the upper platehas moved by a distance bb' which is equal to . Now Noting that for solids the shear stress isproportional tostrain while for fluids it isproportional to rateof strain, , which in turn is defined as
.Substituting for we have Since is proportional to , wehave or It is found that for common fluids such as air, waterand oil therelationship between shear stress and velocitygradient can beexpressed as,
The constant of proportionality is an important property offluids in determining the flow behaviour and is calledDynamic Viscosity or AbsoluteViscosity. It is usual to refer to it as justViscosity. It has the dimensions andunits of in the SI system. Fluids for which the shear stress varies linearly as rate ofstrain are called Newtonian Fluids. Many of the commonfluids belong to this category- air, water. When the relationshipbetween shear stress and rate of strain is not linear, the fluidis designated Non-Newtonian. Examples of thiscategory are some of the industrial fluids such as plastics,sludge and biological fluids such as blood. Typical plots of shearstress vs rate of strain are shown in Fig.1.5. Rheologyis the branch of fluid mechanics which specialises in thesefluids. We consider primarily common fluids such as water and airand hence restrict ourselves to Newtonian fluids. Viscosity of a fluid is strongly dependent on temperature and is aweak function of pressure. For example, when the pressure of airis increased from 1 atm to 50 atm, its viscosity increases only byabout 10 percent allowing one to ignore its dependence onpressure. Fig.1.6 shows the manner of dependence ofviscosity on temperature for some of the common fluids. It is seenthat the viscosity of liquids deceases with temperature while thatfor the gases increases with temperature. This difference inbehaviour is explained by the cohesive and intermolecular forceswithin the fluid. Liquids are characterized by strong cohesiveforces and close packing of molecules. When temperature increasescohesive forces are weakened and there is less resistance tomotion. Hence viscosity decreases. With gases, the cohesive forcesare very weak and the molecules are spaced apart. Viscosity is dueto the exchange of momentum between molecules as a result ofrandom motion. As the temperature increases the molecular activityincreases giving rise to an increased resistance to motion or inother words viscosity increases.
University of Sydney |
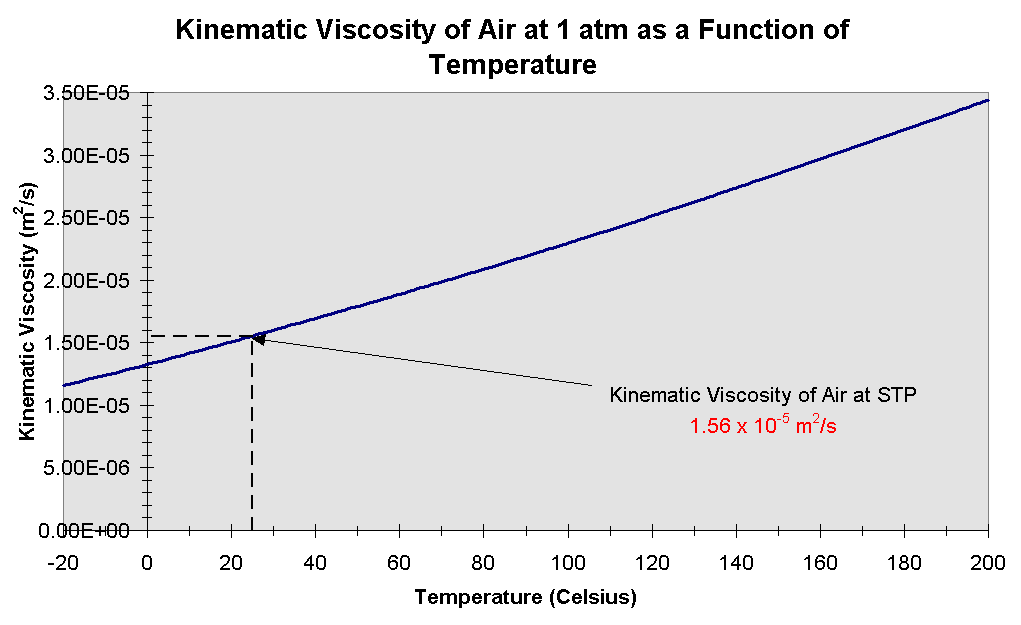
Online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of air at temperatures ranging from -100 to 1600°C (-150 to 2900°F) at pressure ranging from 1 to 10 000 bara (14.5 - 145000 psia) - SI and Imperial Units
The viscosity of a fluid is a measure of its resistance to gradual deformation by shear stress or tensile stress.

For further definitions, go to Absolute (dynamic) and kinematic viscosity. Absolute or dynamic viscosity is used to calculate Reynold's Number to determine if a fluid flow is laminar, transient or turbulent.
Tabulated values and viscosity units conversion are given below the figures.
Online Air Viscosity Calculator
Viscosity Of Air
The calculator below can be used to calculate air dynamic or kinematic viscosity at given temperatures and atmospheric pressure.
The output dynamic viscosity is given as Pa*s, N*s/m2, cP, mPa*s, lbf*s/ft2 and lbm/(ft*h),
while the kinematic viscosity is given as cSt, m2/s, and ft2/s
Note! Temperature must be within the ranges -100 - 1600 °C, -150 - 2900 °F, 175 - 1900 K and 310-3400 °R to get valid values.
Related Topics
- Gases and Compressed Air - Air, LNG, LPG and other common gas properties, pipeline capacities, sizing of relief valves
- Material Properties - Material properties for gases, fluids and solids - densities, specific heats, viscosities and more
- Viscosity - Documents giving viscosity of different kind of chemical species at varying conditions
- Air Psychrometrics - The study of moist and humid air - psychrometric charts, Mollier diagrams, air-condition temperatures and absolute and relative humidity and moisture content
- Fluid Mechanics - The study of fluids - liquids and gases. Involves velocity, pressure, density and temperature as functions of space and time
Related Documents
- Absolute or Dynamic Viscosity Online Converter - Convert between dynamic or absolute viscosity units - Poiseuille, Poise, centPoise and more
- Absolute, Dynamic and Kinematic Viscosity - Dynamic, absolute and kinematic viscosities - convert between CentiStokes (cSt), centipoises (cP), Saybolt Universal Seconds (SSU) and degree Engler
- Air - Composition and Molecular Weight - Dry air is a mechanical mixture of nitrogen, oxygen, argon and several other gases in minor amounts
- Air - Density at varying pressure and constant temperatures - Figures and tables showing changes in air density at pressure varying from 1 to 10 000 bara (14.5 - 145000 psi) and constant, selected temperatures. Figures are given in different scales.
- Air - Density, Specific Weight and Thermal Expansion Coefficient at Varying Temperature and Constant Pressures - Online calculator, figures and tables showing density, specific weight and thermal expansion coefficient of air at temperatures ranging -100 to 1600 °C (-140 to 2900 °F) at atmospheric and higher pressure - Imperial and SI Units
- Air - Diffusion Coefficients of Gases in Excess of Air - Diffusion coefficients, D12, for gases in large excess of air at temperature varying from 0 - 400 °C
- Air - Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight (or molar mass) can be calculated by adding the weight of each component
- Air - Prandtl Number - Figures and table showing changes in Prandtl number for air with changes in temperature and pressure
- Air - Properties at Gas-Liquid Equilibrium Conditions - Figures and tables showing how the properties of air changes along the boiling and condensation curves (temperature and pressure between triple point and critical point conditions). An air phase diagram are also given.
- Air - Specific Heat at Constant Pressure and Varying Temperature - Online calculator, figures and tables showing how specific heat (Cp and Cv) of dry air vary with temperature at different pressures, SI and imperial units
- Air - Specific Heat at Constant Temperature and Varying Pressure - Figures and table showing isobaric (Cp) and isochoric (Cv) specific heat of air at constant temperature and varying pressure ranging 0.01 to 10000 bara
- Air - Thermal Conductivity - Online calculator, figures and tables showing air thermal conductivity at varying temperature and pressure, SI and imperial units.
- Air - Thermal Diffusivity - Figures and tables showing dry air thermal diffusivity at varying temperarure and pressure, SI and Imperial units
- Air - Thermophysical Properties - Thermal properties of air - density, viscosity, critical temperature and pressure, triple point, enthalpi and entropi, thermal conductivity and diffusicity, and more
- Air Conditioning - Cooling of Air and Generated Condensate - Water may condensate when air is cooled in an air conditioning system
- Air Humidity Ratio - The ratio between the mass of water vapor present in moist air - to the mass of dry air
- Air Solubility in Water - Amount of air that can be dissolved in water - decreases with temperature and increases with pressure
- Air- Moisture Holding Capacity vs. Temperature - Moisture holding capacity of air increases with temperature
- Benzene - Dynamic and Kinematic Viscosity - Online calculator, figures and table showing dynamic and kinematic viscosity of benzene, C6H6, at varying temperature and pressure - Imperial and SI Units
- Butane - Dynamic and Kinematic Viscosity - Online calculators, figures and tables showing dynamic and kinematic viscosity of liquid and gaseous butane, C4H10, at varying temperarure and pressure, SI and Imperial units
- Compressed Air Pressure Drop Diagrams - Metric Units - Pressure drop in compressed air pipe lines
- Dry Air Properties - Dry air properties at temperatures ranging 175 - 1900 K - specific heat, ratio of specific heats, dynamic viscosity, thermal conductivity, Prandtl number, density and kinematic viscosity
- Dynamic or Absolute Viscosity Converting Chart - Dynamic viscosity converting chart with units like Poiseuille - Poise - centiPoise and more
- Ethanol - Dynamic and Kinematic Viscosity - Online calculator, figures and tables showing dynamic and kinematic viscosity of ethanol, C2H5OH, at varying temperature and pressure - Imperial and SI Units
- Ethylene - Dynamic and Kinematic Viscosity - Online calculator, figures and tables showing dynamic and kinematic viscosity of ethylene, C2H4, also called ethene or acetene, at varying temperature and pressure - Imperial and SI Units
- Kinematic Viscosity - Online Converter - Convert between different kinematic viscosity units - centistokes, poise, lentor and more
- Methane - Dynamic and Kinematic Viscosity - Online calculator, figures and tables showing dynamic and kinematic viscosity of methane, CH4, at varying temperature and pressure - Imperial and SI Units
- Oxygen - Dynamic and Kinematic Viscosity - Online calculator, figures and tables showing dynamic and kinematic viscosity of oxygen, O2, at varying temperature and pressure - Imperial and SI Units
- Propane - Dynamic and Kinematic Viscosity - Online calculators, figures and tables showing dynamic and kinematic viscosity of liquid and gaseous propane at varying temperarure and pressure, SI and Imperial units
- Specific Volume of Moist Air - Specific volume is defined as the total volume of humid air per mass unit of dry air
- Steam Viscosity - Absolute viscosity of steam at pressure ranging 1 - 10000 psia
- Viscosity Converting Chart - Convert between the viscosity units Centiposes, milliPascal, CentiStokes and SSU
- Water Vapor in Air - Weight of water vapor in air
Viscosity Of Air 70f
Viscosity Of Air At 20 C
Tag Search
Viscosity Of Air And Water
- en: air viscosity dynamic absolute kinematic temperature
- es: viscosidad cinemática del aire temperatura absoluta dinámica
- de: Luft Viskosität dynamisch absoluten kinematischen Temperatur